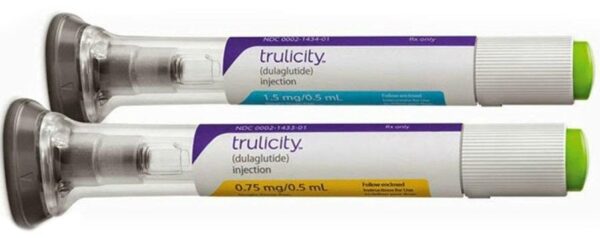
Dulaglutide Trulicity Injection, Prescription, Treatment: Diabetes And Weight Loss
Price range: $300.00 through $3,300.00
- Premium Quality
- Secure Payments
- Satisfaction Guarantee
- Worldwide Shipping
- Money Back Guarantee
| Packaging Size |
2 PEN PER BOX
|
| Brand |
TRULICITY
|
| Manufacturer |
LILLY
|
| Composition |
DULAGLUTIDE
|
| Treatment |
DIABETES AND WEIGHT LOSS
|
| Prescription/Non prescription |
Prescription
|
| Shelf Life |
2 YEAR
|
| Form |
Injection
|
Description
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
TRULICITY® is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated (1):
- As an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus.
- To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
Limitations of Use:
DOSAGE AND ADMINISTRATION
Adult Dosage (2.1)
- Recommended starting dosage is 0.75 mg injected subcutaneously once weekly.
- After 4 weeks, the dosage may be increased to 1.5 mg once weekly for additional glycemic control.
- If additional glycemic control is needed, increase dosage in 1.5 mg increments after at least 4 weeks on the current dosage.
- Maximum recommended dosage is 4.5 mg injected subcutaneously once weekly.
Pediatric Dosage (2.2)
- Recommended starting dosage is 0.75 mg injected subcutaneously once weekly.
- If additional glycemic control is needed, increase dosage to the maximum recommended dosage of 1.5 mg once weekly after at least 4 weeks on the 0.75 mg dosage.
Recommendations Regarding Missed Dose (2.3)
- If a dose is missed, administer the missed dose as soon as possible if there are at least 3 days (72 hours) until the next scheduled dose.
Important Administration Instructions (2.4)
- Administer once weekly at any time of day with or without food.
- Inject subcutaneously in the abdomen, thigh, or upper arm.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Thyroid C-cell Tumors: See Boxed Warning (5.1).
- Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2).
- Hypoglycemia: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing the dose of insulin secretagogue or insulin may be necessary (5.3).
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema) have occurred. Discontinue TRULICITY and promptly seek medical advice (5.4).
- Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions (5.5).
- Severe Gastrointestinal Adverse Reactions: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients (5.6).
- Diabetic Retinopathy Complications: Have been reported in a cardiovascular outcomes trial. Monitor patients with a history of diabetic retinopathy (5.7).
- Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated (5.8).
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Has been reported in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures. Instruct patients to inform healthcare providers of any planned surgeries or procedures (5.9).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥5%) are nausea, diarrhea, vomiting, abdominal pain, and decreased appetite (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: Should be used during pregnancy only if the potential benefit justifies the potential risk to fetus (8.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Additional information
| Quantity | 10 pens, 30 pens, 60 pens, 90 pens, 120 pens |
|---|

Reviews
There are no reviews yet.